Advenza Bacillus clausii (AVZ 7911™): A Proven Probiotic Strain with Preclinical Toxicological Studies manufactured in a WHO cGMP certified facility.
Antibiotics wipe out infection and your good gut bacteria too. That’s why you need a probiotic like AVZ 7911™ — which helps protect one from infection and restore the gut balance.
In the competitive landscape of probiotics, Bacillus clausii is known for its proven benefits in supporting gut health, preventing antibiotic-associated diarrhoea, and boosting immunity. However, while Bacillus clausii is widely used, only select strains demonstrate the superior efficacy required for optimal clinical and commercial performance. Introducing, Bacillus clausii -AVZ 7911™, a distinct, clinically advanced proprietary strain developed by Advenza, which is redefining the standards for probiotic performance — especially during antibiotic treatment.
The biggest challenge for probiotics administered alongside antibiotics is simple: survival. Most probiotics are destroyed by the very antibiotics prescribed to treat infections. But AVZ 7911™ is different. It is a poly-antibiotic resistant strain, and endures the onslaught created by several of the most popular antibiotics.
AVZ 7911™ is uniquely resistant to a broad range of antibiotics, including:
- Penicillin
- Cephalosporins
- Tetracycline
- Macrolides
- Aminoglycosides
- Novobiocin
- Chloramphenicol
- Thiamphenicol
- Lincomycin
- Isoniazid
- Cycloserine
- Rifampicin
- Nalidixic acid
- Pipemidic acid
This poly-antibiotic resistance means AVZ 7911™ can be safely and effectively administered during antibiotic treatment — without being wiped out by the very medications it’s meant to support.
Why AVZ 7911™ ?
Delivers the essential characteristics of a high-performance probiotic:
- Preclinical Toxicological Studies – Advenza Bacillus clausii AVZ 7911™ is a no risk strain based on the preclinical toxicology studies conducted by Advenza as per OECD guidelines.
- Scientifically Proven Safety – Non-pathogenic and safe for use in both humans and animals, supported by rigorous scientific testing.
- Survives Harsh Digestive Conditions – Naturally acid stable, and withstand stomach acids and bile, ensuring it reaches the intestines alive and active.
- Heat Stable – Maintains its viability across a wide range of temperatures, making it ideal for global distribution and storage.
- Shelf-Stable Without Refrigeration – As a spore-forming bacterium, AVZ 7911™ offers long-term stability without the need for cold storage.
💡 This combination of poly-antibiotic resistance, safety and stability makes AVZ 7911™ one of the most effective & trusted Bacillus clausii strains available in the market.
Manufacturing Excellence: WHO cGMP-certified, Fully Integrated Manufacturing Facility at Vapi
- End to End Integration
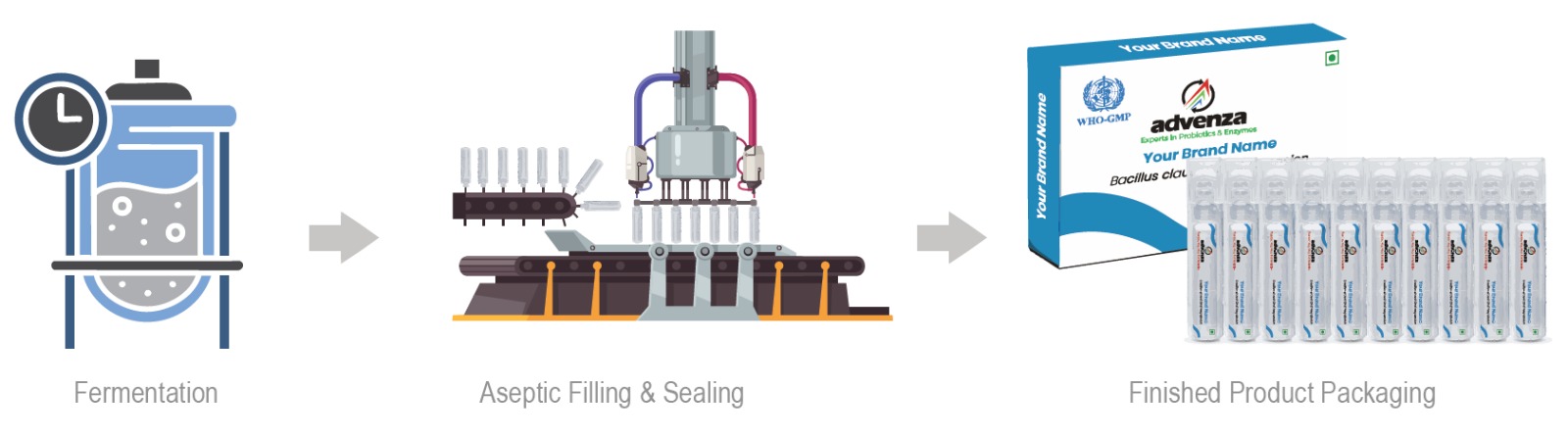
The core strength of Advenza lies in complete manufacturing Integration at it’s own facility – i.e. from fermented raw material, to intermediate concentrate, to finished product – every stage being monitored thoroughly, and aseptically controlled for quality & consistency. - Technical Expertise
Supported and backed up by a technical team having 40+ years of experience & expertise in fermentation and pharma manufacturing - State-of-the-Art WHO cGMP Certified Facility
Advenza’s facilities are proudly WHO cGMP certified, guaranteeing a high level of adherance to global production & quality norms, thus inspiring tremendous confidence in our partners worldwide. - High Throughput Capacity
Advenza’s facility is well capable of producing >100 million vials per annum


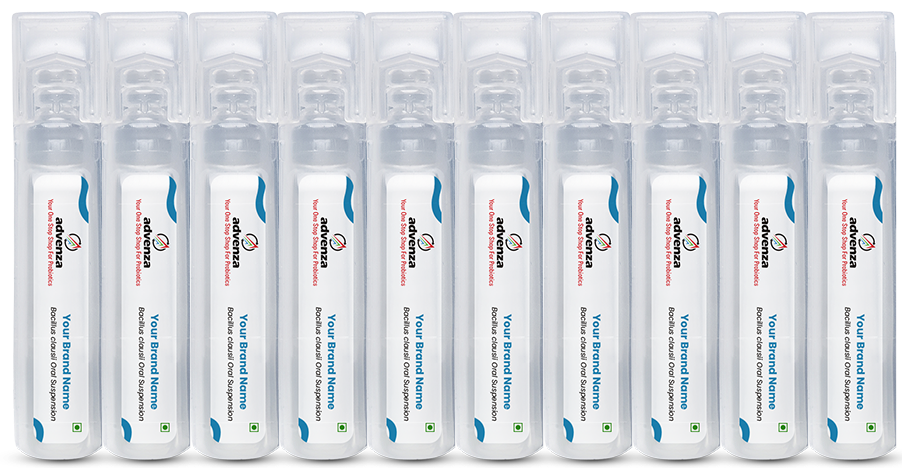
Key Highlights of Advenza’s Bacillus clausii AVZ 7911™ Vials:
- Typical Indications: Prevention & Treatment of Diarrhea
- Potencies Available: 2 billion CFU / 4 billion CFU per vial
- Pack Size: Set of 10 contiguous vials
- Key Features of Advenza’s Vials:
- Hermetic Seal: Vials are sealed containers that provide an excellent barrier against external contaminants and maintain the sterility of the contents. This makes them suitable for storing and delivering sensitive medications and supplements.
- Tamper-Proof: Vials are tamper-proof, as they need to be broken to access the contents. This ensures the integrity of the product and prevents tampering.
- Accurate Dosage: Vials typically contain pre-measured doses of medication, making it easy to ensure accurate dosing.
- Long Shelf Life: The hermetic seal of Vials helps extend the shelf life of the contents by protecting them from degradation due to exposure to air, light, or moisture.
- Sturdy Construction: Our Vials are made of LDPE which is inert and doesn’t react with most medications, ensuring the purity and stability of the contents.
Why Advenza? The End-to-End Integration Advantage
Unlike most suppliers who outsource parts of their value chain, Advenza has end to end integration & control over it’s entire production value chain in-house:
- No Reliance on Third-Party Suppliers for Probiotic Raw Materials
- Focus on Quality, Stability, Consistency and Traceability.
- Short Delivery Time-frames and Optimised Costs
This Integrated model allows Advenza to not only minimise risk, but also enables faster innovation, customized solutions, and stable pricing.
Conclusion
Advenza prides itself in offering One Stop Probiotic Solutions.
Advenza’s Bacillus clausii AVZ 7911™ is not just any other probiotic offering — it delivers simutaneously all the hallmarks of a great product: a clinically-studied safe strain, encapsulated into very high quality vials, and manufactured in an advanced, integrated, WHO cGMP-certified, state-of-the-art facility.
Contact us to know more



